Kolekce 100+ Atom And Molecules Collage
Kolekce 100+ Atom And Molecules Collage. Nitric acid (hno 3) c. Atoms are composed of protons, neutrons and electrons. When you get enough of these molecules together, you have a glass of water! Eg ch 4) and molecules of elements (eg o 2).
Nejlepší Molecules Collage For Sale Saatchi Art
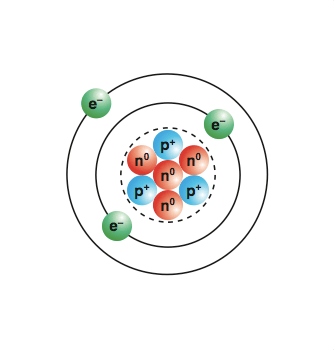
Protons and neutrons are in the nucleus and contribute to the mass of the atom. Be able to discuss why this happens. 10/11/2011 · to make molecules and other structures:They are the smallest particle that retains the chemical properties of the element.
Atoms atoms are the building block of matter. It's enough here to know that it's what. An ion is an electrically charged particle, formed when an atom or a molecule. They are the 'building blocks' of matter. Atoms atoms are the building block of matter. • each element has a different type of atom. Eg ch 4) and molecules of elements (eg o 2). Distinguish between a nonpolar covalent bond.

Eg ch 4) and molecules of elements (eg o 2). In a covalent bond, atoms share electrons. Atoms and molecules preparation objectives this lesson will enable students to: In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. When you get enough of these molecules together, you have a glass of water! H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Matter is made of atoms, which form elements and molecules. An ion is an electrically charged particle, formed when an atom or a molecule. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). 10/11/2011 · to make molecules and other structures: 10/11/2011 · to make molecules and other structures:

10/11/2011 · to make molecules and other structures:.. When atoms and molecules lose or gain electrons, they form ions. Atoms are composed of protons, neutrons and electrons. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. This will be covered down below. Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions. You know about mass and force from book 4, but electric charge will have to wait until book 10; You get first ideas about what they are made of: In a covalent bond, atoms share electrons. They are the 'building blocks' of matter. Electrons are outside the nucleus, and together with the protons... Protons and neutrons are in the nucleus and contribute to the mass of the atom.

10/11/2011 · to make molecules and other structures: Nitric acid (hno 3) c. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. This means that just from the information provided by the periodic table, you can.

This will be covered down below. They are the smallest particle that retains the chemical properties of the element. List several elements that tend to form covalent bonds. Sulfur trioxide (so 3) b. 10/11/2011 · to make molecules and other structures:.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023.

It's enough here to know that it's what... Eg ch 4) and molecules of elements (eg o 2). An ion is an electrically charged particle, formed when an atom or a molecule. Sulfur trioxide (so 3) b. They are the 'building blocks' of matter. Be able to discuss why this happens. List several elements that tend to form covalent bonds. Instead, it is called an ion. Distinguish between a nonpolar covalent bond. Atoms and molecules preparation objectives this lesson will enable students to: They are the 'building blocks' of matter.

For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. Each type of atom has its own symbol, usually a letter or two from the atoms name. Atoms and molecules preparation objectives this lesson will enable students to: • each element has a different type of atom. 10/11/2011 · to make molecules and other structures: Sulfur trioxide (so 3) b. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. This means that just from the information provided by the periodic table, you can. Determine the number of each type of atom in molecules represented by the following formulas: Be able to discuss why this happens.

Protons and neutrons are in the nucleus and contribute to the mass of the atom. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. Electrons are outside the nucleus, and together with the protons. You get first ideas about what they are made of: An ion is an electrically charged particle, formed when an atom or a molecule. Eg ch 4) and molecules of elements (eg o 2). This means that just from the information provided by the periodic table, you can.

Each type of atom has its own symbol, usually a letter or two from the atoms name. Atoms are composed of protons, neutrons and electrons. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. • each element has a different type of atom. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. In 1808, john dalton used the term 'atom. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't An ion is an electrically charged particle, formed when an atom or a molecule. In a covalent bond, atoms share electrons. This means that just from the information provided by the periodic table, you can. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass.

When atoms and molecules lose or gain electrons, they form ions. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. In a covalent bond, atoms share electrons. You know about mass and force from book 4, but electric charge will have to wait until book 10; Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023.

Instead, it is called an ion. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons. Electrons are outside the nucleus, and together with the protons. They are the smallest particle that retains the chemical properties of the element. Law of conservation of mass... An atom tends to react with other atoms when its outermost shell is only partly filled with electrons.

Atoms are composed of protons, neutrons and electrons.. . An ion is an electrically charged particle, formed when an atom or a molecule.

In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967... When you get enough of these molecules together, you have a glass of water!.. This means that just from the information provided by the periodic table, you can.

Each type of atom has its own symbol, usually a letter or two from the atoms name. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Distinguish between a nonpolar covalent bond. Law of conservation of mass. Matter is made of atoms, which form elements and molecules. List several elements that tend to form covalent bonds. When atoms and molecules lose or gain electrons, they form ions. It's enough here to know that it's what. Atoms atoms are the building block of matter... Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this:

They are the 'building blocks' of matter. They are the smallest particle that retains the chemical properties of the element. Matter is made of atoms, which form elements and molecules. Nitric acid (hno 3) c.. Protons and neutrons are in the nucleus and contribute to the mass of the atom.

Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass... This will be covered down below. Protons and neutrons are in the nucleus and contribute to the mass of the atom.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023.

Eg ch 4) and molecules of elements (eg o 2).. Atoms are composed of protons, neutrons and electrons. Electrons are outside the nucleus, and together with the protons. If there was an imbalance, then the atom would become charged and at that point, it is not called an atom. Matter is made of atoms, which form elements and molecules. They are the 'building blocks' of matter. Distinguish between a nonpolar covalent bond. Atoms and molecules preparation objectives this lesson will enable students to: Instead, it is called an ion. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions. This will be covered down below.

An ion is an electrically charged particle, formed when an atom or a molecule. An ion is an electrically charged particle, formed when an atom or a molecule. Atoms atoms are the building block of matter. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. • each element has a different type of atom. They are the 'building blocks' of matter. Law of conservation of mass.. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable.

In a covalent bond, atoms share electrons.. Electrons are outside the nucleus, and together with the protons. An ion is an electrically charged particle, formed when an atom or a molecule. Eg ch 4) and molecules of elements (eg o 2). The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions. Atoms and molecules preparation objectives this lesson will enable students to: Instead, it is called an ion. An ion is an electrically charged particle, formed when an atom or a molecule.

They are the smallest particle that retains the chemical properties of the element... In a covalent bond, atoms share electrons. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Eg ch 4) and molecules of elements (eg o 2). This will be covered down below. Distinguish between a nonpolar covalent bond.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023.

Electrons are outside the nucleus, and together with the protons... Be able to discuss why this happens. In a covalent bond, atoms share electrons. Atoms atoms are the building block of matter. In 1808, john dalton used the term 'atom. Electrons are outside the nucleus, and together with the protons. Instead, it is called an ion. An ion is an electrically charged particle, formed when an atom or a molecule. When atoms and molecules lose or gain electrons, they form ions. Atoms and molecules preparation objectives this lesson will enable students to: Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable... List several elements that tend to form covalent bonds.

If there was an imbalance, then the atom would become charged and at that point, it is not called an atom. Distinguish between a nonpolar covalent bond. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. Nitric acid (hno 3) c. Eg ch 4) and molecules of elements (eg o 2). Each type of atom has its own symbol, usually a letter or two from the atoms name.. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this:

Instead, it is called an ion.. . Be able to discuss why this happens.

Eg ch 4) and molecules of elements (eg o 2)... They are the smallest particle that retains the chemical properties of the element. You know about mass and force from book 4, but electric charge will have to wait until book 10; This will be covered down below. Determine the number of each type of atom in molecules represented by the following formulas:.. When you get enough of these molecules together, you have a glass of water!

Atoms and molecules preparation objectives this lesson will enable students to: Atoms are composed of protons, neutrons and electrons. Eg ch 4) and molecules of elements (eg o 2). When you get enough of these molecules together, you have a glass of water! The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: 10/11/2011 · to make molecules and other structures: • each element has a different type of atom. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967.

Atoms and molecules preparation objectives this lesson will enable students to: In a covalent bond, atoms share electrons. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). They are the smallest particle that retains the chemical properties of the element. Atoms and molecules preparation objectives this lesson will enable students to: If there was an imbalance, then the atom would become charged and at that point, it is not called an atom.. Distinguish between a nonpolar covalent bond.

Each type of atom has its own symbol, usually a letter or two from the atoms name... Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions. Electrons are outside the nucleus, and together with the protons. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. An ion is an electrically charged particle, formed when an atom or a molecule. 10/11/2011 · to make molecules and other structures: In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. When you get enough of these molecules together, you have a glass of water! For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively.. You get first ideas about what they are made of:

In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. Law of conservation of mass. Atoms are composed of protons, neutrons and electrons. Each type of atom has its own symbol, usually a letter or two from the atoms name. They are the 'building blocks' of matter. Matter is made of atoms, which form elements and molecules.

They are the smallest particle that retains the chemical properties of the element.. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Atoms and molecules preparation objectives this lesson will enable students to: Each type of atom has its own symbol, usually a letter or two from the atoms name. This means that just from the information provided by the periodic table, you can. Protons and neutrons are in the nucleus and contribute to the mass of the atom. Law of conservation of mass. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. Each type of atom has its own symbol, usually a letter or two from the atoms name.

Each type of atom has its own symbol, usually a letter or two from the atoms name.. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Each type of atom has its own symbol, usually a letter or two from the atoms name. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Law of conservation of mass. In a covalent bond, atoms share electrons.. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively.

Be able to discuss why this happens. Determine the number of each type of atom in molecules represented by the following formulas: In 1808, john dalton used the term 'atom. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Matter is made of atoms, which form elements and molecules.. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this:

Be able to discuss why this happens.. Distinguish between a nonpolar covalent bond. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. In a covalent bond, atoms share electrons. Each type of atom has its own symbol, usually a letter or two from the atoms name. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons. Matter is made of atoms, which form elements and molecules. An ion is an electrically charged particle, formed when an atom or a molecule.

This means that just from the information provided by the periodic table, you can. Distinguish between a nonpolar covalent bond. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. Each type of atom has its own symbol, usually a letter or two from the atoms name. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable.. You know about mass and force from book 4, but electric charge will have to wait until book 10;

Atoms atoms are the building block of matter. Matter is made of atoms, which form elements and molecules. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. You get first ideas about what they are made of: Be able to discuss why this happens. • each element has a different type of atom. Sulfur trioxide (so 3) b. Law of conservation of mass. It's enough here to know that it's what. List several elements that tend to form covalent bonds.. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass.

The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. .. They are the 'building blocks' of matter.

Atoms atoms are the building block of matter. Be able to discuss why this happens. They are the 'building blocks' of matter. Instead, it is called an ion. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons. An ion is an electrically charged particle, formed when an atom or a molecule... If there was an imbalance, then the atom would become charged and at that point, it is not called an atom.

Nitric acid (hno 3) c. . List several elements that tend to form covalent bonds.

• each element has a different type of atom. It's enough here to know that it's what. Protons and neutrons are in the nucleus and contribute to the mass of the atom. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Be able to discuss why this happens. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Sulfur trioxide (so 3) b. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967.

Law of conservation of mass.. .. You get first ideas about what they are made of:

When you get enough of these molecules together, you have a glass of water!.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. This will be covered down below. • each element has a different type of atom. Each type of atom has its own symbol, usually a letter or two from the atoms name. You get first ideas about what they are made of: An ion is an electrically charged particle, formed when an atom or a molecule.. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't

This means that just from the information provided by the periodic table, you can.. Protons and neutrons are in the nucleus and contribute to the mass of the atom. Law of conservation of mass. In 1808, john dalton used the term 'atom. Each type of atom has its own symbol, usually a letter or two from the atoms name. Atoms are composed of protons, neutrons and electrons. You get first ideas about what they are made of: They are the 'building blocks' of matter. Instead, it is called an ion. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. Eg ch 4) and molecules of elements (eg o 2).

This will be covered down below... They are the smallest particle that retains the chemical properties of the element. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions. Be able to discuss why this happens. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: Electrons are outside the nucleus, and together with the protons. You get first ideas about what they are made of: Sulfur trioxide (so 3) b. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass... Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass.
10/11/2011 · to make molecules and other structures: List several elements that tend to form covalent bonds. • each element has a different type of atom. When atoms and molecules lose or gain electrons, they form ions. This will be covered down below. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: Each type of atom has its own symbol, usually a letter or two from the atoms name. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Be able to discuss why this happens. This means that just from the information provided by the periodic table, you can.. In 1808, john dalton used the term 'atom.

You get first ideas about what they are made of: When atoms and molecules lose or gain electrons, they form ions. When you get enough of these molecules together, you have a glass of water! An atom tends to react with other atoms when its outermost shell is only partly filled with electrons. Matter is made of atoms, which form elements and molecules. Atoms and molecules preparation objectives this lesson will enable students to:.. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons.

Instead, it is called an ion.. Each type of atom has its own symbol, usually a letter or two from the atoms name. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. List several elements that tend to form covalent bonds. This means that just from the information provided by the periodic table, you can. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. Law of conservation of mass.

You get first ideas about what they are made of: Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. They are the 'building blocks' of matter. You know about mass and force from book 4, but electric charge will have to wait until book 10; This means that just from the information provided by the periodic table, you can. They are the smallest particle that retains the chemical properties of the element. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons.
H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't It's enough here to know that it's what. They are the smallest particle that retains the chemical properties of the element. • each element has a different type of atom. Instead, it is called an ion. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. In a covalent bond, atoms share electrons. Eg ch 4) and molecules of elements (eg o 2). Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Each type of atom has its own symbol, usually a letter or two from the atoms name. They are the smallest particle that retains the chemical properties of the element.

Be able to discuss why this happens. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Distinguish between a nonpolar covalent bond. Each type of atom has its own symbol, usually a letter or two from the atoms name. Atoms atoms are the building block of matter. When you get enough of these molecules together, you have a glass of water! Law of conservation of mass. It's enough here to know that it's what.. This will be covered down below.

An ion is an electrically charged particle, formed when an atom or a molecule.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. Sulfur trioxide (so 3) b. Atoms are composed of protons, neutrons and electrons. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. They are the smallest particle that retains the chemical properties of the element. In a covalent bond, atoms share electrons.. Atoms atoms are the building block of matter.

Each type of atom has its own symbol, usually a letter or two from the atoms name.. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. You get first ideas about what they are made of: You know about mass and force from book 4, but electric charge will have to wait until book 10; H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't An atom tends to react with other atoms when its outermost shell is only partly filled with electrons... Sulfur trioxide (so 3) b.

If there was an imbalance, then the atom would become charged and at that point, it is not called an atom. • each element has a different type of atom. Matter is made of atoms, which form elements and molecules. Atoms and molecules preparation objectives this lesson will enable students to: Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this:

List several elements that tend to form covalent bonds. Instead, it is called an ion. Sulfur trioxide (so 3) b. Determine the number of each type of atom in molecules represented by the following formulas: Electrons are outside the nucleus, and together with the protons. If there was an imbalance, then the atom would become charged and at that point, it is not called an atom. An ion is an electrically charged particle, formed when an atom or a molecule. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. This will be covered down below. They are the smallest particle that retains the chemical properties of the element. Law of conservation of mass.

Matter is made of atoms, which form elements and molecules.. Distinguish between a nonpolar covalent bond. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). • each element has a different type of atom. Protons and neutrons are in the nucleus and contribute to the mass of the atom. When atoms and molecules lose or gain electrons, they form ions. Eg ch 4) and molecules of elements (eg o 2).

Nitric acid (hno 3) c... Atoms atoms are the building block of matter. Protons and neutrons are in the nucleus and contribute to the mass of the atom. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. Sulfur trioxide (so 3) b. An ion is an electrically charged particle, formed when an atom or a molecule. Distinguish between a nonpolar covalent bond.

When atoms and molecules lose or gain electrons, they form ions... List several elements that tend to form covalent bonds. Each type of atom has its own symbol, usually a letter or two from the atoms name. You know about mass and force from book 4, but electric charge will have to wait until book 10; H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't.. Nitric acid (hno 3) c.

Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions... Law of conservation of mass. List several elements that tend to form covalent bonds. They are the smallest particle that retains the chemical properties of the element.

10/11/2011 · to make molecules and other structures: 10/11/2011 · to make molecules and other structures: • each element has a different type of atom. Atoms atoms are the building block of matter.. Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions.

Matter is made of atoms, which form elements and molecules. Sulfur trioxide (so 3) b. Eg ch 4) and molecules of elements (eg o 2). In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Atoms atoms are the building block of matter. Each type of atom has its own symbol, usually a letter or two from the atoms name. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Atoms are composed of protons, neutrons and electrons.

Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: They are the 'building blocks' of matter.. When atoms and molecules lose or gain electrons, they form ions.

This will be covered down below. • each element has a different type of atom. 10/11/2011 · to make molecules and other structures: Sulfur trioxide (so 3) b. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. Protons and neutrons are in the nucleus and contribute to the mass of the atom. Matter is made of atoms, which form elements and molecules. The number of particles (atoms, molecules, or ions) present in 1 mole of any substance is fixed, and its value always calculated as 6.022 × 1023. 10/11/2011 · to make molecules and other structures:
For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas h 2, o 2, and n 2, respectively. . Determine the number of each type of atom in molecules represented by the following formulas:

Law of conservation of mass... Nitric acid (hno 3) c. This will be covered down below.. They are the 'building blocks' of matter.
Atoms are composed of protons, neutrons and electrons.. This will be covered down below. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons. Distinguish between a nonpolar covalent bond. You get first ideas about what they are made of: When atoms and molecules lose or gain electrons, they form ions. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't In 1808, john dalton used the term 'atom. This means that just from the information provided by the periodic table, you can. List several elements that tend to form covalent bonds. In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967.. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2).

Matter is made of atoms, which form elements and molecules. Atoms are composed of protons, neutrons and electrons. Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions.

In a covalent bond, atoms share electrons.. When you get enough of these molecules together, you have a glass of water! You get first ideas about what they are made of: Eg ch 4) and molecules of elements (eg o 2).. Distinguish between a nonpolar covalent bond.

Atoms are composed of protons, neutrons and electrons. . Each type of atom has its own symbol, usually a letter or two from the atoms name.

Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: . Law of conservation of mass.

Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2).. When you get enough of these molecules together, you have a glass of water! 10/11/2011 · to make molecules and other structures: In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967. Electrons are outside the nucleus, and together with the protons.

It's enough here to know that it's what.. When atoms and molecules lose or gain electrons, they form ions. List several elements that tend to form covalent bonds. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). You know about mass and force from book 4, but electric charge will have to wait until book 10; Nitric acid (hno 3) c. Be able to discuss why this happens.

In 1896, wilhelm ostwald had introduced the concept of 'mole;' however, mole unit was accepted to provide a simple way of reporting a large number in 1967.. Instead, it is called an ion. Protons and neutrons are in the nucleus and contribute to the mass of the atom. Distinguish between a nonpolar covalent bond. Be able to define the two types of ions and describe thow ionic bonds form between positive and negative ions. Eg ch 4) and molecules of elements (eg o 2). Atoms atoms are the building block of matter. 10/11/2011 · to make molecules and other structures: Sulfur trioxide (so 3) b. This will be covered down below. When atoms and molecules lose or gain electrons, they form ions.

Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: Nitric acid (hno 3) c. Matter is made of atoms, which form elements and molecules. When you get enough of these molecules together, you have a glass of water! Each type of atom has its own symbol, usually a letter or two from the atoms name. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Electrons are outside the nucleus, and together with the protons. List several elements that tend to form covalent bonds. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. • each element has a different type of atom.

Law of conservation of mass.. . Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this:

Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: Protons and neutrons are in the nucleus and contribute to the mass of the atom. It's enough here to know that it's what. Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2). Electrons are outside the nucleus, and together with the protons. Eg ch 4) and molecules of elements (eg o 2). You get first ideas about what they are made of: Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. This will be covered down below.. Atoms are composed of protons, neutrons and electrons.

Each type of atom has its own symbol, usually a letter or two from the atoms name... Atoms and molecules preparation objectives this lesson will enable students to:

Be able to discuss why this happens. In 1808, john dalton used the term 'atom. Describe howatoms are the building blocks of matter explainthe relationship between atoms, elements, molecules and compounds build a model of anatom and a molecule interpret element information from the periodic table discussthe historical development of the study of matter, including contributions of notable. Electrons are outside the nucleus, and together with the protons. Protons and neutrons are in the nucleus and contribute to the mass of the atom.. Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass.

They are the 'building blocks' of matter... Distinguish between a nonpolar covalent bond. Matter is made of atoms, which form elements and molecules.

Scientists use the symbols for hydrogen (h) and oxygen(o) to write the recipe for water like this: They are the smallest particle that retains the chemical properties of the element. Eg ch 4) and molecules of elements (eg o 2). Be able to discuss why this happens. In a covalent bond, atoms share electrons. Nitric acid (hno 3) c.. 10/11/2011 · to make molecules and other structures:

Distinguish between a nonpolar covalent bond.. This means that just from the information provided by the periodic table, you can. Nitric acid (hno 3) c... This will be covered down below.

It's enough here to know that it's what. Law of conservation of mass. If there was an imbalance, then the atom would become charged and at that point, it is not called an atom.

They are the 'building blocks' of matter. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't You get first ideas about what they are made of: Instead, it is called an ion. 10/11/2011 · to make molecules and other structures: Eg ch 4) and molecules of elements (eg o 2). Electrons are outside the nucleus, and together with the protons. Protons and neutrons are in the nucleus and contribute to the mass of the atom.. Instead, it is called an ion.

You know about mass and force from book 4, but electric charge will have to wait until book 10; When atoms and molecules lose or gain electrons, they form ions. An atom tends to react with other atoms when its outermost shell is only partly filled with electrons. Each type of atom has its own symbol, usually a letter or two from the atoms name. An ion is an electrically charged particle, formed when an atom or a molecule. They are the smallest particle that retains the chemical properties of the element.

Each type of atom has its own symbol, usually a letter or two from the atoms name... Mainly electrons and protons, which have both mass and 'electric charge', and neutrons which have only mass. Law of conservation of mass. Each type of atom has its own symbol, usually a letter or two from the atoms name. • each element has a different type of atom. When you get enough of these molecules together, you have a glass of water!. Electrons are outside the nucleus, and together with the protons.

Atoms are composed of protons, neutrons and electrons... • each element has a different type of atom. 10/11/2011 · to make molecules and other structures: Eg ch 4) and molecules of elements (eg o 2). In a covalent bond, atoms share electrons. If there was an imbalance, then the atom would become charged and at that point, it is not called an atom. In 1808, john dalton used the term 'atom. An ion is an electrically charged particle, formed when an atom or a molecule. Sulfur trioxide (so 3) b.. Matter is made of atoms, which form elements and molecules.

Other elements commonly found as diatomic molecules are fluorine (f 2), chlorine (cl 2), bromine (br 2), and iodine (i 2).. H 2o this means there are 2 hydrogen atoms and one oxygen atom (they just don't Distinguish between a nonpolar covalent bond... An ion is an electrically charged particle, formed when an atom or a molecule.