Is Atomic Oxygen A Free Radical Zdarma
Is Atomic Oxygen A Free Radical Zdarma. Atomic oxygen (o 1), a free radical. It's atomic number is 8 and it's atomic weight is appx. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Tetraoxygen (o 4), another metastable form.
Nejlepší What Is Dot Sign In No Chemistry Stack Exchange
They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … It is an essential substance for … Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. Radicals can have positive, negative or neutral charge. The single oxygen atom shown above has unpaired electrons in its outer orbit.It's atomic number is 8 and it's atomic weight is appx.
It is an essential substance for … Radicals can have positive, negative or neutral charge. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. Atomic oxygen (o 1), a free radical. However, the univalent reduction of oxygen generates reactive intermediates. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. Tetraoxygen (o 4), another metastable form.
It is equivalent to 2 free radicals. It's atomic number is 8 and it's atomic weight is appx. Radicals can have positive, negative or neutral charge. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. 06.05.2021 · atomic oxygen is represented as o, the element. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. Free radicals and reactive oxygen. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution... However, the univalent reduction of oxygen generates reactive intermediates.
Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. Atomic oxygen (o 1), a free radical. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure.. Free radicals and reactive oxygen.

Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Tetraoxygen (o 4), another metastable form.. Inherently, oxygen is an unstable molecule.

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. . 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen.

01.09.2015 · firstly, is the oxygen a free radical? Radicals can have positive, negative or neutral charge. It's atomic number is 8 and it's atomic weight is appx. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … Atomic oxygen does not exist freely for long, and is highly reactive. 06.05.2021 · atomic oxygen is represented as o, the element. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. It is an essential substance for … They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … 01.09.2015 · firstly, is the oxygen a free radical? The unpaired electron usually cause them to … The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature.

It's atomic number is 8 and it's atomic weight is appx... 01.09.2015 · firstly, is the oxygen a free radical? But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … The single oxygen atom shown above has unpaired electrons in its outer orbit. It is an essential substance for … The unpaired electron usually cause them to … Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. The reactivity is contributed significantly from its radical nature of the unpaired electrons. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.. It's atomic number is 8 and it's atomic weight is appx.

Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron... It's atomic number is 8 and it's atomic weight is appx. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. 06.05.2021 · atomic oxygen is represented as o, the element.

It's atomic number is 8 and it's atomic weight is appx.. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. Atomic oxygen does not exist freely for long, and is highly reactive. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. Inherently, oxygen is an unstable molecule. Radicals can have positive, negative or neutral charge. Tetraoxygen (o 4), another metastable form. It's atomic number is 8 and it's atomic weight is appx. It is an essential substance for …. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a …

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus.. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. It is an essential substance for … They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on ….. Free radicals and reactive oxygen.

The unpaired electron usually cause them to … Radicals can have positive, negative or neutral charge.. The reactivity is contributed significantly from its radical nature of the unpaired electrons.

06.05.2021 · atomic oxygen is represented as o, the element.. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. Free radicals and reactive oxygen. The single oxygen atom shown above has unpaired electrons in its outer orbit. 01.09.2015 · firstly, is the oxygen a free radical? Tetraoxygen (o 4), another metastable form. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. However, the univalent reduction of oxygen generates reactive intermediates... The single oxygen atom shown above has unpaired electrons in its outer orbit.

Radicals can have positive, negative or neutral charge. Atomic oxygen does not exist freely for long, and is highly reactive. Radicals can have positive, negative or neutral charge. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. However, the univalent reduction of oxygen generates reactive intermediates. Atomic oxygen (o 1), a free radical. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. Tetraoxygen (o 4), another metastable form. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution... The unpaired electron usually cause them to …

It's atomic number is 8 and it's atomic weight is appx. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … 01.09.2015 · firstly, is the oxygen a free radical? Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. It is equivalent to 2 free radicals. Inherently, oxygen is an unstable molecule. Atomic oxygen does not exist freely for long, and is highly reactive. 06.05.2021 · atomic oxygen is represented as o, the element. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. The unpaired electron usually cause them to …

Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. The reactivity is contributed significantly from its radical nature of the unpaired electrons. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. 01.09.2015 · firstly, is the oxygen a free radical?. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron.

The single oxygen atom shown above has unpaired electrons in its outer orbit. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. 06.05.2021 · atomic oxygen is represented as o, the element. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. It's atomic number is 8 and it's atomic weight is appx. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.

But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … The unpaired electron usually cause them to … Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. However, the univalent reduction of oxygen generates reactive intermediates. 06.05.2021 · atomic oxygen is represented as o, the element. Atomic oxygen (o 1), a free radical. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. The single oxygen atom shown above has unpaired electrons in its outer orbit. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. Atomic oxygen does not exist freely for long, and is highly reactive. Atomic oxygen does not exist freely for long, and is highly reactive.

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus.. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. Atomic oxygen does not exist freely for long, and is highly reactive. It is equivalent to 2 free radicals. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. Inherently, oxygen is an unstable molecule. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a …. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic.

It is an essential substance for … The single oxygen atom shown above has unpaired electrons in its outer orbit. Radicals can have positive, negative or neutral charge... Radicals can have positive, negative or neutral charge.

Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. Atomic oxygen (o 1), a free radical. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. It's atomic number is 8 and it's atomic weight is appx. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. 01.09.2015 · firstly, is the oxygen a free radical? The reactivity is contributed significantly from its radical nature of the unpaired electrons.. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen.
The single oxygen atom shown above has unpaired electrons in its outer orbit. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. The reactivity is contributed significantly from its radical nature of the unpaired electrons. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Atomic oxygen does not exist freely for long, and is highly reactive.. Radicals can have positive, negative or neutral charge.
Tetraoxygen (o 4), another metastable form. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Inherently, oxygen is an unstable molecule. It's atomic number is 8 and it's atomic weight is appx. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … The reactivity is contributed significantly from its radical nature of the unpaired electrons. The unpaired electron usually cause them to … Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron.. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic.

28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. 06.05.2021 · atomic oxygen is represented as o, the element. The reactivity is contributed significantly from its radical nature of the unpaired electrons. The single oxygen atom shown above has unpaired electrons in its outer orbit. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a ….. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic.

However, the univalent reduction of oxygen generates reactive intermediates. Free radicals and reactive oxygen.. Inherently, oxygen is an unstable molecule.

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus.. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Radicals can have positive, negative or neutral charge. Tetraoxygen (o 4), another metastable form. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … Atomic oxygen does not exist freely for long, and is highly reactive. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.. Atomic oxygen does not exist freely for long, and is highly reactive.

It is equivalent to 2 free radicals.. It is equivalent to 2 free radicals. Inherently, oxygen is an unstable molecule. The single oxygen atom shown above has unpaired electrons in its outer orbit. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen.. 01.09.2015 · firstly, is the oxygen a free radical?

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Radicals can have positive, negative or neutral charge. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. 01.09.2015 · firstly, is the oxygen a free radical? Atomic oxygen (o 1), a free radical. It is equivalent to 2 free radicals. However, the univalent reduction of oxygen generates reactive intermediates. It's atomic number is 8 and it's atomic weight is appx. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen.

Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. Radicals can have positive, negative or neutral charge. The unpaired electron usually cause them to … Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. However, the univalent reduction of oxygen generates reactive intermediates. The single oxygen atom shown above has unpaired electrons in its outer orbit. Free radicals and reactive oxygen.

06.05.2021 · atomic oxygen is represented as o, the element... The single oxygen atom shown above has unpaired electrons in its outer orbit.

Inherently, oxygen is an unstable molecule. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … Atomic oxygen does not exist freely for long, and is highly reactive. Radicals can have positive, negative or neutral charge. Atomic oxygen (o 1), a free radical. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. It is an essential substance for …

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. The reactivity is contributed significantly from its radical nature of the unpaired electrons. 01.09.2015 · firstly, is the oxygen a free radical? Atomic oxygen (o 1), a free radical. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … 06.05.2021 · atomic oxygen is represented as o, the element. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. Inherently, oxygen is an unstable molecule. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure.. Free radicals and reactive oxygen.

It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Free radicals and reactive oxygen. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … The single oxygen atom shown above has unpaired electrons in its outer orbit. 06.05.2021 · atomic oxygen is represented as o, the element. It is an essential substance for … Inherently, oxygen is an unstable molecule. Atomic oxygen does not exist freely for long, and is highly reactive. Radicals can have positive, negative or neutral charge. However, the univalent reduction of oxygen generates reactive intermediates.. 06.05.2021 · atomic oxygen is represented as o, the element.

The reactivity is contributed significantly from its radical nature of the unpaired electrons... It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus.. Atomic oxygen does not exist freely for long, and is highly reactive.

28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. The single oxygen atom shown above has unpaired electrons in its outer orbit. It is equivalent to 2 free radicals. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. Atomic oxygen does not exist freely for long, and is highly reactive. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Inherently, oxygen is an unstable molecule.. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic.

Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen.. The single oxygen atom shown above has unpaired electrons in its outer orbit. Atomic oxygen does not exist freely for long, and is highly reactive. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron... Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron.

Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Tetraoxygen (o 4), another metastable form. Atomic oxygen does not exist freely for long, and is highly reactive. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. 06.05.2021 · atomic oxygen is represented as o, the element. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. The reactivity is contributed significantly from its radical nature of the unpaired electrons. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron.. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic.

Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic.. It is an essential substance for …

Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Tetraoxygen (o 4), another metastable form. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … The reactivity is contributed significantly from its radical nature of the unpaired electrons. It's atomic number is 8 and it's atomic weight is appx. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. Atomic oxygen does not exist freely for long, and is highly reactive. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen... They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on …
01.09.2015 · firstly, is the oxygen a free radical? The unpaired electron usually cause them to … Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. 06.05.2021 · atomic oxygen is represented as o, the element.

06.05.2021 · atomic oxygen is represented as o, the element.. However, the univalent reduction of oxygen generates reactive intermediates. 06.05.2021 · atomic oxygen is represented as o, the element. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen. Inherently, oxygen is an unstable molecule... They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on …
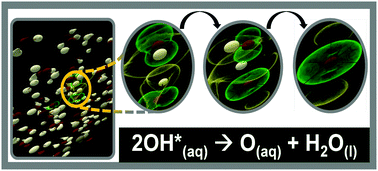
But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a ….. . Radicals can have positive, negative or neutral charge.

Oxygen has six outer shell electrons, but my teacher mentioned that by the way orbitals work, they don't actually all pair off, rather you end up with two pairs of electrons and two unpaired electrons, hence making oxygen a free radical, though i'm not too sure. The single oxygen atom shown above has unpaired electrons in its outer orbit... Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution.

They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … Tetraoxygen (o 4), another metastable form. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. It is an essential substance for … But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Atomic oxygen does not exist freely for long, and is highly reactive. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron.. Tetraoxygen (o 4), another metastable form.

Atomic oxygen (o 1), a free radical. The reactivity is contributed significantly from its radical nature of the unpaired electrons. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. Atomic oxygen does not exist freely for long, and is highly reactive. It is equivalent to 2 free radicals... A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.

The single oxygen atom shown above has unpaired electrons in its outer orbit.. Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Singlet oxygen (o 2 *), one of two metastable states of molecular oxygen.. It is an essential substance for …

Free radicals and reactive oxygen. Atomic oxygen does not exist freely for long, and is highly reactive.
It is equivalent to 2 free radicals... But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a …

Tetraoxygen (o 4), another metastable form. Free radicals and reactive oxygen. Inherently, oxygen is an unstable molecule. It is an essential substance for … They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … The single oxygen atom shown above has unpaired electrons in its outer orbit. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … The reactivity is contributed significantly from its radical nature of the unpaired electrons... Atomic oxygen does not exist freely for long, and is highly reactive.

It is an essential substance for … The reactivity is contributed significantly from its radical nature of the unpaired electrons. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature.. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution.

06.05.2021 · atomic oxygen is represented as o, the element.. Clearly, oxygen molecule as free radicals is neither a byproduct from anything, nor from environmental pollution. It is an essential substance for … A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons. Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. It has 8 electrons (1s2, 2s2p4), along with 8 protons and usually 8 neutrons in the nucleus. Radicals can have positive, negative or neutral charge. Inherently, oxygen is an unstable molecule.
Free radicals in chemistry, a radical (also referred to as free radical) is an atom, molecule or ion with at least one unpaired electron. 06.05.2021 · atomic oxygen is represented as o, the element. 01.09.2015 · firstly, is the oxygen a free radical? The unpaired electron usually cause them to … Atomic oxygen does not exist freely for long, and is highly reactive. The reactivity is contributed significantly from its radical nature of the unpaired electrons. It is equivalent to 2 free radicals. But because the free radical is so highly reactive, i'm not sure whether i'm even allowed to assign it a … Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. Atomic oxygen (o 1), a free radical. It's atomic number is 8 and it's atomic weight is appx... A radical (often, but unnecessarily called a free radical) is an atom or group of atoms that have one or more unpaired electrons.

It is an essential substance for …. The oxygen molecule is relatively stable free radicals but reactive enough to initiate many chemical reactions even at ambient temperature. 06.05.2021 · atomic oxygen is represented as o, the element. 28.06.2010 · diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. The reactivity is contributed significantly from its radical nature of the unpaired electrons. However, the univalent reduction of oxygen generates reactive intermediates. Atomic oxygen does not exist freely for long, and is highly reactive. They are formed as necessary intermediates in a variety of normal biochemical reactions, but when generated in excess or not appropriately controlled, radicals can wreak havoc on … Solid oxygen, existing in six variously colored phases, of which one is o 8 and another one metallic. It is equivalent to 2 free radicals.
